What Family of Proteins Do Tcr Belong to?
Introduction
Wiskott-Aldrich syndrome (WAS) is a severe X-linked chief immunodeficiency caused by the mutations of the WAS gene on the 10-chromosome. WAS is characterized by thrombocytopenia, eczema, increased susceptibility to infection and increased risk to develop autoimmune disease (Gallego et al., 1997; Bosticardo et al., 2009; Massaad et al., 2013). The WAS cistron encodes the Wiskott-Aldrich syndrome protein (WASp), which is of the actin nucleation-promoting factor family. WASp is a cytosolic protein comprising 502 amino acids lacking intrinsic catalytic action. Instead, WASp acts equally scaffold protein that transduces a wide range of signals from cell surface receptors to mediate dynamic changes in the actin cytoskeleton in response to external stimuli (Sun et al., 2019). WASp is expressed exclusively in the hematopoietic cell lineages including T cells, B cells, natural killer (NK) cells, dendritic cells, macrophages, and platelets (Matalon et al., 2013). T cells isolated from WAS patient have a defect in actin reorganization in response to TCR-mediated stimulation (Gallego et al., 1997; Matalon et al., 2013). Furthermore, WASp has an essential office in betoken transduction and effector functions of T cells.
Wiskott-Aldrich syndrome protein belongs to the WASp family unit of proteins consisting of WASp, neuronal (N)-WASp, and Wave 1–3 and WASp and SCAR homolog (WASH) (Linardopoulou et al., 2007). Although, WASp and N-WASp share more fifty% sequence homology also every bit having similar protein binding partners and basic functions, these two proteins are non entirely redundant (Fried et al., 2014). In contrast to WASp, Northward-WASp is widely expressed in multiple tissues (Miki et al., 1996).
In this article, we review recent findings of WASp in TCR-mediated betoken transduction. Since the adaptor poly peptide non-catalytic region of tyrosine kinase (Nck) binds to WASp, we discuss the mechanisms of Nck-mediated WASp recruitment to the TCR whereby information technology may regulate the actin mechanism and signal transduction in close proximity to the TCR.
WASp Structure and Its Change During T Cell Activation
From the North- to the C-terminus, WASp contains a WASp homology i (WH1) domain (also known every bit EVH1, for ENA/VASP homology), a bones (B) domain, a GTPase-bounden domain (GBD), a proline-rich domain (PRD), and a verprolin homology (V), cofilin (C) homology, and acidic region (A) (VCA domain) at the C-terminus (Zhang et al., 2009; Effigy 1A). These distinct domains are required to mediate downstream signaling by binding to unlike cytoskeleton-regulating poly peptide partners. More than twenty protein binding partners accept been reported (Thrasher and Burns, 2010). Although, the structures of the WASp family members vary, the VCA domain is particularly conserved. The VCA domain interacts with actin and Arp2/3, whereas the PRD domain binds to various SH3 domain-containing proteins.
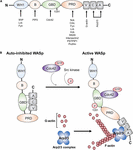
Figure 1. Functional domains in WASp and WASp activation. (A) WASp contains various domains, which can bind proteins involved in TCR-mediated actin cytoskeleton remodeling and betoken transduction. (B) WASp is in a closed motorcar-inhibited conformation in resting state due to its intracellular interaction between GBD and VCA domains. Upon TCR engagement, it mediates the binding of GTP-Cdc42 to the GBD domain, thereby releasing the VCA domain from GBD domain and irresolute the WASp structure into an opened conformation. In improver, WASp tyrosine 291 within GBD can be phosphorylated by the Src family kinases Fyn and Lck. Recruitment of GTP-Cdc42 to GBD and phosphorylation of WASp tyrosine 291 inside GBD consequence in WASp activation. Later on, Arp2/three and monomeric actin can bind the VCA domain, which induces a new actin branch. This figure was modified from Matalon et al. (2013). Wiskott-Aldrich syndrome protein–dynamic regulation of actin homeostasis: from activation through function and signal termination in T lymphocytes. Immunol. Rev. 256(i), 10–29; with a permission from John Wiley and Sons; license number 5065241058992.
The first N-last WH1 domain is a binding site for a proline echo motif nowadays in the WASp interacting protein (WIP) (Volkman et al., 2002). WASp is constitutively associated with WIP. WIP regulates WASp activity and promotes WASp stability in resting T cell by protecting WASp from deposition by calpain and proteasome. It is also critical for localizing WASp to areas of actin polymerization (Chou et al., 2006; de la Fuente et al., 2007). In addition, the WH1 domain may deed every bit a binding site for the Src family kinase Fyn and Lck in T cells (Sato et al., 2011; Matalon et al., 2013). Following the WH1 domain, the B domain is involved in the regulation of the WASp conformation, as it can bind the phosphoinositide PIP2 (phosphatidylinositol-four,v-bisphosphate) and acts in couple with the pocket-sized GTPase Cdc42 to release of WASp from its auto-inhibited conformation toward the active conformation (Higgs and Pollard, 2000; Thrasher and Burns, 2010). The GBD domain can collaborate in cis with the C-concluding VCA domain, thereby inducing the closed autoinhibitory conformation (Effigy 1B; Kim et al., 2000). Upon activation, the VCA domain is released from GBD every bit a consequence of the bounden of GTP-bound Cdc42 to the WASp GBD. The PRD serves every bit the docking site for multiple protein binding partners that comprise an SH3 domain such equally Src and Tec family tyrosine kinases (Bunnell et al., 1996; Torres and Rosen, 2006) and the adaptor protein Nck (Rivera et al., 2004; Barda-Saad et al., 2005). Finally, after TCR appointment and the recruitment of GTP-bound Cdc42 to the WASp GBD, the released VCA region can interact with both monomeric actin (through the 5 region) and with the actin-related protein complex Arp2/3 (through the CA region) that work together to stimulate nucleation of branched actin filaments (Figure 1B; Symons et al., 1996; Miki and Takenawa, 1998; Blanchoin et al., 2000; Krause et al., 2000).
In resting T cells, WASp is mainly present in an autoinhibited conformation in the cytoplasm, in which the VCA domain interacts with a hydrophobic patch located within the GBD (Figure 1B). Upon TCR-mediated signaling, the kinase Zeta-associated poly peptide of 70 kDa (ZAP-lxx) is recruited to the TCR and activated. Subsequently, it phosphorylates the adaptor protein Src homology 2 (SH2) domain-containing leukocyte protein of 76 kDa (SLP-76) that has a binding site for Nck and the guanine nucleotide exchange factor Vav-1. Nck is constitutively associated with WASp via the C-terminal SH3 domain of Nck binding to the PRD of WASp (Rivero-Lezcano et al., 1995; Paensuwan et al., 2015). Thus, Nck acts every bit a bridge to recruit WASp to the SLP-76 signaling complex. In association with SLP-76, Vav-i mediates the exchange of GDP- to GTP-jump Cdc42, Rho family GTPases. GTP-spring Cdc42 then interacts with the WASp GBD, thereby releasing WASp from its auto-inhibited conformation, assuasive VCA to bind to the Arp2/three complex. In one case bound to the VCA domain, Arp2/three promotes the branching of the actin polymerization and rearrangement at the T cell-APC contact site (Zeng et al., 2003; Matalon et al., 2013). In addition, tyrosine 291 within WASp GBD can be phosphorylated by the Src family kinases Fyn and Lck (Badour et al., 2004; Torres and Rosen, 2006), which interact with the WASp WH1 domain (Sato et al., 2011). Phosphorylation of tyrosine 291 is essential for WASp activation (Cory et al., 2002; Badour et al., 2004). Thus, besides the recruitment of Cdc42 to WASp, WASp activation can exist indicated by tyrosine 291 phosphorylation. Interestingly, phosphorylation of Y291 within WASp GBD domain besides mediates WASp degradation by calpain and proteasome proteolysis (Watanabe et al., 2013; Sunday et al., 2019).
Initiation and Following Pathways of TCR Signaling
Following TCR date by its ligand peptide-MHC (pMHC), the TCR and its signaling molecules quickly form microclusters where signaling is amplified and sustained (Seminario and Bunnell, 2008; Choudhuri and Dustin, 2010). The TCR is composed of the pMHC-binding TCRαβ heterodimer non-covalently associated with the non-variable betoken transduction subunits CD3εγ, CD3εδ, and CD3ζζ (Kane et al., 2000; Alarcón et al., 2003). The TCRα and TCRβ chains accept curt cytoplasmic tails with no intrinsic capacity to mediate signal transduction. In contrast, the CD3 molecules serve every bit signal transducers by transferring information of TCR pMHC-bounden to initiate signaling transduction (Hayes et al., 2003; Schamel et al., 2019). Each of the CD3 signaling subunits has cytoplasmic immunoreceptor tyrosine-based activation motifs (ITAMs) (Reth, 1989), one present in CD3ε, CD3δ, and CD3γ and three in CD3ζ. In addition, CD3ε has a proline-rich sequence (PRS) (Gil et al., 2002) and the receptor kinase (RK) motif (Hartl et al., 2020), which are required to regulate TCR activation. We volition draw these 2 meaning motifs in the next section.
T prison cell receptor date results in phosphorylation on tyrosine residues within the ITAMs, creating pairs of phosphotyrosines, which serve as docking sites for proteins containing SH2 domains such every bit ZAP-70. Post-obit the binding of ZAP-70 to ITAMs, ZAP-70 phosphorylates LAT (linker for activation of T cells). Phosphorylated LAT recruits various enzymes and adaptor proteins to form multi-protein signaling complexes (Zhang et al., 1998). Recently, the formins mDia1 and mDia3 play a crucial role in F-actin polymerization, which facilitate LAT phosphorylation by ZAP-seventy (Thumkeo et al., 2020). Phosphorylated LAT tin can bind to phospholipase Cγ1 (PLCγ1), phosphoinositide 3-kinase (PI3K), growth factor receptor-jump protein 2 (Grb2) and GRB2-related adaptor downstream of Shc (Gads). Gads serves as a bridge to recruit SLP-76 to phospho-LAT, where SLP-76 is phosphorylated by ZAP-lxx. Every bit described above, WASp is recruited to SLP-76 through Nck, where WASp can associate and activate Arp2/3 to promote actin filament formation (Wunderlich et al., 1999; Barda-Saad et al., 2005).
After the signaling complexes take formed, they are required to activate four pathways: Ras/Rac-mitogen-activated protein kinase (MAPK), protein kinase C (PKC), nuclear factor of κB (NF-κB) and Catwo+-mediated signaling pathways, such equally the nuclear factor of activated T cells (NFAT) pathway. The Ras pathway activates the extracellular receptor-activated kinase (Erk), a fellow member of the MAP kinase family. The activated Erk translocates to the nucleus and phosphorylates its substrate Elk1. Phospho-Elk1 and so stimulates the transcription of c-Fos, a component of the transcription gene the activation protein one (AP-ane). In parallel with the Ras pathway, Rac is activated by Vav-i, thereby generating Rac-GTP. The active Rac-GTP activates another MAP kinase called c-Jun Northward-terminal kinase (Jnk). In one case activation, Jnk and then phosphorylates c-Jun, the second component of AP-1 (Smith-Garvin et al., 2009; Conley et al., 2016).
PLCγ1 spring to phospho-LAT is phosphorylated past ZAP-70 and the Tec family kinase Itk. Phosphorylated PLCγ1 catalyzes the hydrolysis of the plasma membrane phospholipid PIP2 generating two breakup products, membrane-bound diacylglycerol (DAG) and inositol 1,4,five-trisphosphate (IP3) (Rhee and Bae, 1997; Braiman et al., 2006). DAG leads to the activation of PKCθ, which then mediates the activation and nuclear translocation of NF-κB. IP3 stimulates the increment in intracellular Ca2+, which subsequently activates the transcription factor NFAT to translocate to the nucleus. In the nucleus, the transcription factors AP-ane, NF-κB and NFAT bind to promotors of specific genes (Smith-Garvin et al., 2009). In the side by side section, we hash out WASp's part in proximal TCR signaling.
WASp'due south Role in Proximal TCR Signaling
Assembly of WASp to SLP-76 signalosome upon TCR engagement is a cardinal stride for WASp to mediate the branching of the actin cytoskeleton polymerization (Wunderlich et al., 1999; Barda-Saad et al., 2005). Furthermore, two more than pathways of WASp recruitment to distinct cellular compartments in the vicinity of TCR-CD3 have been reported and are the points to be discussed in this section.
Ligand-binding to the TCR leads to the stabilization of the agile conformation of the TCR (Gil et al., 2002; Minguet et al., 2007; Lee et al., 2015), in which the PRS, the RK motif and the ITAMs become exposed (Swamy et al., 2016; Hartl et al., 2020). In the active conformation the CD3ε PRS binds to the Due north-terminal SH3 domain of Nck, thus recruiting Nck to the TCR (Gil et al., 2002). Our contempo work has suggested a role of actin polymerization in decision-making Nck recruitment to CD3ε PRS (Wipa et al., 2020), although the verbal mechanism remains enigmatic. At the aforementioned time Lck is recruited to the RK motif and the ITAMs tin exist phosphorylated (Hartl et al., 2020). Phosphorylation of CD3ε at the second ITAM tyrosine stabilizes the Nck-CD3ε interaction, since the SH2 domain of Nck tin bind to the phospho-tyrosine (Paensuwan et al., 2016). This leaves the C-terminal SH3 domain of Nck costless to collaborate with WASp. It has been establish that WASp is constitutively associated with both Nck isoforms, Nck1 and Nck2, and both isoforms can acquaintance with CD3ε (Gil et al., 2002). Indeed, we institute that WASp can be recruited to the TCR upon TCR stimulation (Paensuwan et al., 2015; Figure ii), and this might be mediated by Nck. Thus, it was postulated that the directly recruitment of WASp to TCR-CD3 may also bring the effector molecules to TCR-CD3 that are essential for decision-making the actin reorganization and betoken transduction (Ngoenkam et al., 2018; Figure 2). Among these, GTP-Cdc42 has been found to be recruited to the T cell:APC contact site, where TCR, WASp and Vav-i are accumulated (Cannon et al., 2001; Yokosuka and Saito, 2010). Localization of these proteins at the T prison cell:APC contact site supports the hypothesis of molecular machinery driven the actin polymerization at TCR. Further, recruitment of WASp to the TCR was relied on both the CD3 conformational modify and fractional CD3ε tyrosine phosphorylation (Paensuwan et al., 2015). This finding is coincident with the pattern of Nck recruitment to CD3ε following TCR triggering. This strengthens the possibility of Nck to mediate the recruitment of WASp to CD3ε. Further piece of work is needed to assess the relative contributions of TCR-recruited WASp in regulating T prison cell activation. This finding may reveal the additional function of WASp as well its role in regulating actin polymerization such every bit controlling bespeak transduction in close proximity to the TCR.
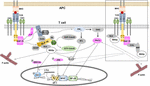
Effigy 2. The role of WASp in bespeak transduction in T cells. TCR ligation with pMHC triggers signal transduction within the cytoplasm of T cells. Once phosphorylated by ZAP-seventy, phospho-LAT recruits proteins to form the LAT/SLP-76 signalosome containing Nck and Vav. Vav activates the Rho GTPase Cdc42, which in turn interacts with WASp. This complex is recruited to the signalosome via Nck. In clan with GTP-Cdc42, WASp is released from the auto-inhibited conformation and then initiates the ARP2/three-dependent F-actin polymerization. In Ras/Rac-MAPK pathway, WASp is essential for the translocation of phospho-Erk1/ii into the nucleus. Once in the nucleus, phospho-Erk1/2 phosphorylates Elk1, which and so causes c-fos expression, a component of transcription factor AP-1. WASp was not required for the activation of Jnk, a kinase causing c-jun expression that is the 2d component of AP-1. In the PKC pathway, WASp contributes to PKCθ activation in response to depression doses of antigen. Activated PKCθ is required for activation of transcription gene NF-κB and its nuclear translocation. For Ca2+-mediated signaling, WASp is indispensable for intracellular Caii+ mobilization, which is essential for NFAT translocation to the nucleus. In the squared box, two alternative pathways of WASp recruitment to TCR are shown. Firstly, WASp is constitutively associated with Nck and Nck-WASp is recruited to the TCR upon TCR ligation. Secondly, preformed CrkL-WIP-WASp is recruited to ZAP-70 following T prison cell activation. We propose that these two recent identified pools of WASp play a role in controlling actin polymerization and may contribute to the nuclear translocation of phospho-Erk1/2, PKCθ-mediated NF-κB activation and Caii+ influx.
Since WASp too binds to Lck (Matalon et al., 2013) recruitment of WASp to the TCR might aid in phosphorylating the TCR. However, in WASp-deficient T cells phosphorylation of CD3ζ and ZAP-70 and total tyrosine proteins was undisturbed (Zhang et al., 1999), arguing against an important role of WASp in CD3 phosphorylation. Information technology has been found in resting T cells that WASp is complexed with WIP (Ramesh et al., 1997), which direct interacts with the adaptor poly peptide CrkL [CT10 regulator of kinase (Crk)-like] to form a CrkL-WIP-WASP circuitous. Following TCR ligation, CrkL, WIP and WASP were co-precipitated with ZAP-70 (Sasahara et al., 2002). Thus, a CrkL-WIP-WASP complex is recruited to ZAP-70 in response to TCR date to generate a ZAP-70-CrkL-WIP-WASp circuitous (Sasahara et al., 2002). Recruitment of this complex to the IS is mediated by the association of the CrkL SH2 domain to a phospho-tyrosine within interdomain B region of ZAP-70 (Chan et al., 1992). PKCθ and then phosphorylates WIP thereby releasing WASp from WIP-mediated inhibition and then WASp is activated past membrane bound Cdc42 resulting in actin polymerization (Sasahara et al., 2002). Thus, besides via a SLP-76/Nck and a TCR/Nck complex, an alternative pathway of WASp recruitment to the IS is mediated by ZAP-70.
WASp's Role in the Ras/Rac-MAPK Pathway
In mammalian cells, there are three major members of MAPKs including Erk (Schaeffer and Weber, 1999), the p38 (Han and Ulevitch, 1999), and Jnk (Davis, 2000). Erk can exist activated by Ras, while p38 and Jnk are activated by Rho family unit GTPases, including Rac and Cdc42 (Rincón et al., 2001). The nuclear target of these 3 MAP kinase members is the activation of the transcription cistron AP-1.
T cells isolated from both WAS patient and WASp deficient (−/−) mice were unable to secrete IL-2 in response to TCR stimulation (Molina et al., 1993; Zhang et al., 1999; Cannon and Burkhardt, 2004). This impairment was not associated with TCR-proximal signaling since tyrosine phosphorylation of CD3ζ, ZAP-70, and total cellular protein detected in TCR-stimulated WAS – / – murine T cells were similar to those observed in the wild-type counterparts (Zhang et al., 1999). The IL-2 promotor is activated past the transcription factors NFAT, AP-1 and NF-κB. The activity of AP-1 and expression of c-Fos, but not c-Jun were markedly impaired in T cells from WASp– / – mice upon TCR stimulation (Cianferoni et al., 2005). Interestingly, upstream signaling proteins of AP-1 including Jnk, c-Jun, and Erk were normally phosphorylated. These results are consistent with previous studies showing that murine WAS – / – T cells exhibited normal phosphorylation of Erk and Jnk (Zhang et al., 1999). All the same, translocation of phosphorylated Erk into the nucleus and phosphorylation of its nuclear substrate, Elk1 were impaired. This phosphorylated nuclear Elk is responsible for activation of c-Fos, a component of the transcription factor AP-1 (Cianferoni et al., 2005). Thus, WASp is essential but for nuclear translocation of phospho-Erk, Elk1 phosphorylation and expression of c-Fos.
WASp'southward Function in PKC-Mediated Signaling
T cells limited dissimilar PKC isoforms, including PKC-α, δ, ϵ, η, θ, and ζ (Brezar et al., 2015). PKCθ is the most studied isoform and is the outset PKC family member that is recruited to the IS (Arendt et al., 2002). PKCθ plays a primal role in a range of signaling cascades that ultimately leads to activation of downstream transcription factors NF-κB, NFAT, and AP-1 (Baier-Bitterlich et al., 1996; Sun et al., 2000; Pfeifhofer et al., 2003Berg-Brown et al., 2004; Hayashi and Altman, 2007). In addition, PKCθ has been postulated to be involved TCR-induced actin polymerization through a process possibly mediated past WIP (Sasahara et al., 2002; Krzewski et al., 2006).
In one case a ZAP-70-CrkL-WIP-WASp complex is formed at IS, PKCθ phosphorylates on WIP. This allows WASp to disengage from the WIP-WASp complex and to proceed its activation and function. Equally reported, PKCθ activation is dependent on Lck, Vav-1, ZAP-lxx, and SLP-76 (Sasahara et al., 2002). Thus, it has been proposed that the ZAP-70-CrkL-WIP pathway and PKCθ are the cardinal players upstream of WASp activation (Sasahara et al., 2002). In keeping with this hypothesis, information technology was found that WASp-deficient T cells in response to antigen-specific APCs showed normal PKCθ polarization to the IS and stimulation of these cells with low doses of antigen caused macerated polarization of PKCθ (Cannon and Burkhardt, 2004), whereas activation with all peptide doses dumb IL-2 production in these cells. Thus, WASp is suggested to be the protein that tin lower the threshold for organizing PKCθ at the IS. In addition, the roles of WASp on IS formation is proposed to be independent of its role in IL-2 product (Cannon and Burkhardt, 2004).
The data of WASp function in activation of NF-κB is scarce. Even so, information technology has been institute that nuclear translocation and action of NF-κB were normal in T cells from WASp–/– mice, whereas NFAT dephosphorylation and nuclear localization, nuclear AP-1 bounden action, and expression of c-Fos were all impaired (Cianferoni et al., 2005). Moreover, in the T helper ane (Th1)-skewed cells, mutation of the VCA domain that is required for Arp2/3-dependent F-actin polymerization did not affect NF-κB-p65 nuclear translocation (Sadhukhan et al., 2014). Together, WASp is required for PKCθ activation in response to low doses of antigen, but WASp is not required NF-κB activation in T cells.
WASp's Role in Caii+-Mediated Signaling Pathways
T prison cell receptor engagement triggers the mobilization of Caii+, which is required for T cell activation, gene expression, motility, synapse formation, cytotoxicity, evolution, and differentiation (Feske, 2007). One time entered into the cells, Ca2+ activates calcineurin resulting in nuclear translocation of NFAT (Gwack et al., 2007). Peripheral lymphocytes exclusively express NFAT-1, NFAT-2, and NFAT-4 (Amasaki et al., 1998). T cells from WAS patients and WASp-deficient mice show defects in IL-2 production upon TCR-induced T cell activation. This was associated with a partial reduction in intracellular Catwo+ mobilization compared with wild-blazon cells, while phosphorylation of CD3ζ, ZAP-70, and Erk was normal (Zhang et al., 1999; Badour et al., 2004; Cannon and Burkhardt, 2004). WASp is required for the assembly of the IS structure, which is essential for optimal sustained calcium signaling (Calvez et al., 2011).
Consistent with mouse WASp-deficient T cells, CD4+ and CD8+ T cells from WAS patients secrete low levels of IL-2 equally well as IFN-γ, and TNF-α in response to stimulation with anti-CD3 and anti-CD28 antibiotic (Trifari et al., 2006). Defective cytokine product is associated with the reduction of nuclear translocation of NFAT-one in CD4+ T cells, while a NFAT-1 and NFAT-2 reduction was observed in CD8+ T cells (Trifari et al., 2006). Previous reports show that the WH1 domain of WASp plays an important role in TCR-induced NFAT-mediated transcription (Silvin et al., 2001). It has been proposed that WASp WH1 perhaps binds WIP to initiate transcriptional NFAT activation. In improver, mutation of WASp WH2 domain (besides known as the verprolin domain), which is essential for Arp2/three-mediated actin polymerization, did not inhibit NFAT activation. Thus, it is suggested that WASp is indispensable for NFAT activation in a manner that is contained of its part in Arp2/3-induced actin polymerization (Silvin et al., 2001). WASp may indirectly regulate TCR-mediated NFAT activation through bounden with adaptor protein Nck, which afterward binds the Pak serine/threonine kinase to promote TCR-mediated NFAT activation (Yablonski et al., 1998).
Conclusion Remarks
Since WASp has been identified, accumulating pieces of bear witness reveal the role of WASp every bit a nucleation-promoting factor that transform signals from cell surface receptors to actin cytoskeleton rearrangement. Intense research has uncovered the functional domains of WASp as well as its interacting partners, near of which are involved in controlling actin-filament formation. Previous model of WASp recruitment to IS following TCR ligation is relied on SLP-76/Nck complex. However, ii alternative pathways of WASp recruitment to IS were identified which are mediated by TCR-Nck and ZAP-70-CrkL-WIP. These findings reveal different WASp puddle existing in T cells and which pool contributing to regulate actin polymerization is yet opened for investigation. The essential office of WASp in actin-dependent T jail cell activation has been continually reported. However, fiddling is known near WASp in TCR-mediated signal transduction and T cell activation. WASp-scarce cells show normal in TCR-proximal signaling such equally phosphorylation of CD3ζ and ZAP-70. However, they show a defect in nuclear translocation of phospho-Erk and decreased intracellular Ca2+ mobilization, the exact crusade of which is not known. Diminishing these signaling events results in an impairment of AP-ane and NFAT activation and reduced of cytokine production. WASp is not involved translocation of Jnk and NF-κB. Interestingly, contained roles of WASp in NFAT activation and actin polymerization have been proposed. Thus, understanding of the role played by WASp in TCR signaling is one of the recent scientific interests to improve the knowledge of etiology of WASp and the treatment of WAS.
Author Contributions
JN collected the data and drafted the manuscript. PW and PP helped in retrieving the data. WS and SP supervised and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
SP has received research grants from the Thailand Science Inquiry and Innovations (TSRI, grant no. BRG6180010) and National Science, Research and Innovation Fund (NSRF, grant nos. R2564B011, R2564B012, and R2565B001). JN received research grants from Naresuan University (no. R2559B064), and the Thailand Enquiry Fund (TRG5880030). This study was also supported past the High german Research Foundation (DFG) through BIOSS—EXC294 and CIBSS—EXC 2189 to WS, SFB854 (B19 to WS) and SFB1381 (A9 to WS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could exist construed as a potential conflict of involvement.
References
Alarcón, B., Gil, D., Delgado, P., and Schamel, W. W. (2003). Initiation of TCR signaling: regulation within CD3 dimers. Immunol. Rev. 191, 38–46. doi: 10.1034/j.1600-065x.2003.00017.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Amasaki, Y., Masuda, E. S., Imamura, R., Arai, Grand., and Arai, North. (1998). Singled-out NFAT family proteins are involved in the nuclear NFAT-Deoxyribonucleic acid binding complexes from human thymocyte subsets. J. Immunol. 160, 2324–2333.
Google Scholar
Arendt, C. W., Albrecht, B., Soos, T. J., and Littman, D. R. (2002). Poly peptide kinase C-theta: signalling from the center of the T-cell synapse. Curr. Opin. Immunol. xiv, 323–330. doi: 10.1016/s0952-7915(02)00346-1
CrossRef Full Text | Google Scholar
Badour, One thousand., Zhang, J., Shi, F., Leng, Y., Collins, M., and Siminovitch, M. A. (2004). Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 199, 99–112. doi: 10.1084/jem.20030976
PubMed Abstract | CrossRef Full Text | Google Scholar
Baier-Bitterlich, Thou., Uberall, F., Bauer, B., Fresser, F., Wachter, H., Grunicke, H., et al. (1996). Protein kinase C-theta isoenzyme selective stimulation of the transcription factor complex AP-one in T lymphocytes. Mol. Cell. Biol. sixteen, 1842–1850. doi: 10.1128/mcb.16.4.1842
PubMed Abstract | CrossRef Full Text | Google Scholar
Barda-Saad, K., Braiman, A., Titerence, R., Bunnell, South. C., Barr, V. A., and Samelson, L. Due east. (2005). Dynamic molecular interactions linking the T jail cell antigen receptor to the actin cytoskeleton. Nat. Immunol. 6, fourscore–89. doi: x.1038/ni1143
PubMed Abstract | CrossRef Full Text | Google Scholar
Berg-Brown, North. Due north., Gronski, One thousand. A., Jones, R. G., Elford, A. R., Deenick, East. One thousand., Odermatt, B., et al. (2004). PKCtheta signals activation versus tolerance in vivo. J. Exp. Med. 199, 743–752. doi: 10.1084/jem.20031022
PubMed Abstract | CrossRef Full Text | Google Scholar
Blanchoin, L., Amann, K. J., Higgs, H. Due north., Marchand, J. B., Kaiser, D. A., and Pollard, T. D. (2000). Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404, 1007–1011. doi: ten.1038/35010008
PubMed Abstract | CrossRef Total Text | Google Scholar
Bosticardo, Chiliad., Marangoni, F., Aiuti, A., Villa, A., and Roncarolo, Thou. G. (2009). Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood 113, 6288–6295. doi: x.1182/blood-2008-12-115253
PubMed Abstract | CrossRef Full Text | Google Scholar
Braiman, A., Barda−Saad, 1000., Sommers, C. L., and Samelson, L. Eastward. (2006). Recruitment and activation of PLC gamma 1 in T cells: a new insight into sometime domains. EMBO J. 25, 774–784. doi: 10.1038/sj.emboj.7600978
PubMed Abstruse | CrossRef Total Text | Google Scholar
Bunnell, S. C., Henry, P. A., Kolluri, R., Kirchhausen, T., Rickles, R. J., and Berg, L. J. (1996). Identification of Itk/Tsk Src homology 3 domain ligands. J. Biol. Chem. 271, 25646–25656. doi: 10.1074/jbc.271.41.25646
PubMed Abstract | CrossRef Total Text | Google Scholar
Calvez, R., Lafouresse, F., De Meester, J., Galy, A., Valitutti, S., and Dupré, L. (2011). The Wiskott-Aldrich syndrome protein permits associates of a focused immunological synapse enabling sustained T-prison cell receptor signalling. Haematologica 96, 1415–1423. doi: 10.3324/haematol.2011.040204
PubMed Abstract | CrossRef Full Text | Google Scholar
Cannon, J. Fifty., and Burkhardt, J. K. (2004). Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J. Immunol. 173, 1658–1662. doi: ten.4049/jimmunol.173.iii.1658
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cannon, J. L., Labno, C. M., Bosco, Grand., Seth, A., McGavin, 1000. H. K., Siminovitch, K. A., et al. (2001). WASP recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity 15, 249–259. doi: x.1016/S1074-7613(01)00178-9
CrossRef Full Text | Google Scholar
Chan, A. C., Iwashima, M., Turck, C. Due west., and Weiss, A. (1992). ZAP- lxx: a lxx kd protein-tyrosine kinase that associates with the TCR zeta chain. Jail cell 71, 649–662. doi: 10.1016/0092-8674(92)90598-seven
CrossRef Full Text | Google Scholar
Chou, H. C., Antón, I. M., Holt, M. R., Curcio, C., Lanzardo, S., Worth, A., et al. (2006). WIP regulates the stability and localization of WASP to podosomes in migrating dendritic cells. Curr. Biol. xvi, 2337–2344. doi: x.1016/j.cub.2006.x.037
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cianferoni, A., Massaad, Thou., Feske, South., de la Fuente, M. A., Gallego, 50., Ramesh, N., et al. (2005). Lacking nuclear translocation of nuclear cistron of activated T cells and extracellular signal-regulated kinase underlies deficient IL-2 gene expression in Wiskott-Aldrich syndrome. J. Allergy Clin. Immunol. 116, 1364–1371. doi: 10.1016/j.jaci.2005.09.006
PubMed Abstruse | CrossRef Full Text | Google Scholar
Conley, J. 1000., Gallagher, Thou. P., and Berg, L. J. (2016). T cells and gene regulation: the switching on and turning up of genes later on T prison cell receptor stimulation in CD8 T cells. Front. Immunol. 7:76. doi: ten.3389/fimmu.2016.00076
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cory, Yard. O., Garg, R., Cramer, R., and Ridley, A. J. (2002). Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. J. Biol. Chem. 277, 45115–45121. doi: 10.1074/jbc.M203346200
PubMed Abstract | CrossRef Full Text | Google Scholar
Davis, R. J. (2000). Signal transduction by the JNK grouping of MAP kinases. Jail cell 103, 239–252. doi: 10.1016/s0092-8674(00)00116-1
CrossRef Total Text | Google Scholar
de la Fuente, Chiliad. A., Sasahara, Y., Calamito, M., Antón, I. Grand., Elkhal, A., Gallego, Chiliad. D., et al. (2007). WIP is a chaperone for Wiskott-Aldrich syndrome protein (WASP). Proc. Natl. Acad. Sci. U. S. A. 104, 926–931. doi: 10.1073/pnas.0610275104
PubMed Abstract | CrossRef Total Text | Google Scholar
Gallego, M. D., Santamaria, M., Pena, J., and Molina, I. J. (1997). Defective actin reorganization and polymerization of Wiskott-Aldrich T cells in response to CD3-mediated stimulation. Blood 90, 3089–3097.
Google Scholar
Gil, D., Schamel, W. Due west., Montoya, M., Sánchez-Madrid, F., and Alarcón, B. (2002). Recruitment of Nck past CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Prison cell 109, 901–912. doi: 10.1016/s0092-8674(02)00799-7
CrossRef Full Text | Google Scholar
Gwack, Y., Feske, Due south., Srikanth, S., Hogan, P. G., and Rao, A. (2007). Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium 42, 145–156. doi: x.1016/j.ceca.2007.03.007
PubMed Abstract | CrossRef Full Text | Google Scholar
Hartl, F. A., Beck-Garcìa, E., Woessner, N. M., Flachsmann, Fifty. J., Cárdenas, R. M. V., Brandl, South. G., et al. (2020). Noncanonical binding of Lck to CD3ε promotes TCR signaling and Motorcar function. Nat. Immunol. 21, 902–913. doi: ten.1038/s41590-020-0732-three
PubMed Abstract | CrossRef Total Text | Google Scholar
Hayes, South. M., Shores, E. W., and Honey, P. E. (2003). An architectural perspective on signalling by the pre-, alphabeta and gammadelta T cell receptors. Immunol. Rev. 191, 28–37. doi: ten.1034/j.1600-065x.2003.00011.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Higgs, H. N., and Pollard, T. D. (2000). Activation by Cdc42 and PIP(two) of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation past Arp2/3 complex. J. Cell. Biol. 150, 1311–1320. doi: 10.1083/jcb.150.6.1311
PubMed Abstract | CrossRef Full Text | Google Scholar
Kane, L. P., Lin, J., and Weiss, A. (2000). Indicate transduction by the TCR for antigen. Curr. Opin. Immunol. 12, 242–249. doi: ten.1016/s0952-7915(00)00083-2
CrossRef Full Text | Google Scholar
Kim, A. S., Kakalis, L. T., Abdul-Manan, Due north., Liu, 1000. A., and Rosen, Thousand. K. (2000). Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404, 151–158. doi: x.1038/35004513
PubMed Abstract | CrossRef Full Text | Google Scholar
Krause, Grand., Sechi, A. S., Konradt, M., Monner, D., Gertler, F. B., and Wehland, J. (2000). Fyn-bounden protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/three complex link T prison cell receptor (TCR) signalling to the actin cytoskeleton. J. Jail cell. Biol. 149, 181–194. doi: x.1083/jcb.149.1.181
PubMed Abstract | CrossRef Full Text | Google Scholar
Krzewski, G., Chen, X., Orangish, J. S., and Strominger, J. 50. (2006). Germination of a WIP-, WASp-, actin-, and myosin IIA-containing multiprotein complex in activated NK cells and its alteration by KIR inhibitory signalling. J. Cell Biol. 173, 121–132. doi: ten.1083/jcb.200509076
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, M. S., Glassman, C. R., Deshpande, North. R., Badgandi, H. B., Parrish, H. L., Uttamapinant, C., et al. (2015). A mechanical switch couples T cell receptor triggering to the cytoplasmic juxtamembrane regions of CD3ζζ. Immunity 43, 227–239. doi: 10.1016/j.immuni.2015.06.018
PubMed Abstract | CrossRef Total Text | Google Scholar
Linardopoulou, E. V., Parghi, S. Due south., Friedman, C., Osborn, Thou. Eastward., Parkhurst, S. M., and Trask, B. J. (2007). Man subtelomeric Launder genes encode a new subclass of the WASP family. PLoS Genet. three:e237. doi: x.1371/journal.pgen.0030237
PubMed Abstruse | CrossRef Full Text | Google Scholar
Matalon, O., Reicher, B., and Barda-Saad, M. (2013). Wiskott-Aldrich syndrome protein–dynamic regulation of actin homeostasis: from activation through function and signal termination in T lymphocytes. Immunol. Rev. 256, 10–29. doi: x.1111/imr.12112
PubMed Abstract | CrossRef Full Text | Google Scholar
Miki, H., Miura, Chiliad., and Takenawa, T. (1996). N-WASP, a novel actin-depolymerizing poly peptide, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent style downstream of tyrosine kinases. EMBO J. 15, 5326–5335.
Google Scholar
Miki, H., and Takenawa, T. (1998). Direct bounden of the verprolin-homology domain in N-WASP to actin is essential for cytoskeletal reorganization. Biochem. Biophys. Res. Commun. 243, 73–78. doi: ten.1006/bbrc.1997.8064
PubMed Abstract | CrossRef Total Text | Google Scholar
Minguet, S., Swamy, M., Alarcón, B., Luescher, I. F., and Schamel, Westward. Westward. (2007). Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity 26, 43–54. doi: 10.1016/j.immuni.2006.x.019
PubMed Abstract | CrossRef Total Text | Google Scholar
Molina, I. J., Sancho, J., Terhorst, C., Rosen, F. S., and Remold-O'Donnell, Eastward. (1993). T cells of patients with the Wiskott-Aldrich syndrome have a restricted defect in proliferative responses. J. Immunol. 151, 4383–4390.
Google Scholar
Paensuwan, P., Hartl, F. A., Yousefi, O. S., Ngoenkam, J., Wipa, P., Brook-Garcia, E., et al. (2016). Nck binds to the T jail cell antigen receptor using its SH3.1 and SH2 domains in a cooperative manner, promoting TCR performance. J. Immunol. 196, 448–458. doi: 10.4049/jimmunol.1500958
PubMed Abstract | CrossRef Full Text | Google Scholar
Paensuwan, P., Ngoenkam, J., Khamsri, B., Preechanukul, K., Sanguansermsri, D., and Pongcharoen, S. (2015). Evidence for inducible recruitment of Wiskott-Aldrich syndrome protein to T prison cell receptor-CD3 complex in Jurkat T cells. Asian Pac. J. Allergy Immunol. 33, 189–195. doi: 10.12932/AP0544.33.3.2015
PubMed Abstract | CrossRef Full Text | Google Scholar
Pfeifhofer, C., Kofler, K., Gruber, T., Tabrizi, N. Yard., Lutz, C., Maly, Chiliad., et al. (2003). Protein kinase C θ affects Ca2+ mobilization and NFAT activation in primary mouse T cells. J. Exp. Med. 197, 1525–1535. doi: x.1084/jem.20020234
PubMed Abstract | CrossRef Total Text | Google Scholar
Ramesh, North., Antón, I. Thousand., Hartwig, J. H., and Geha, R. Due south. (1997). WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl. Acad. Sci. U. Due south. A. 94, 14671–14676. doi: 10.1073/pnas.94.26.14671
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rivera, G. Thou., Briceño, C. A., Takeshima, F., Snapper, S. B., and Mayer, B. J. (2004). Inducible clustering of membrane-targeted SH3 domains of the adaptor protein Nck triggers localized actin polymerization. Curr. Biol. 14, 11–22. doi: 10.1016/j.cub.2003.12.033
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rivero-Lezcano, O. Thousand., Marcilla, A., Sameshima, J. H., and Robbins, One thousand. C. (1995). Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains. Mol. Cell. Biol. fifteen, 5725–5731. doi: 10.1128/mcb.15.x.5725
PubMed Abstract | CrossRef Full Text | Google Scholar
Sadhukhan, S., Sarkar, 1000., Taylor, G., Candotti, F., and Vyas, Y. Grand. (2014). Nuclear role of WASp in cistron transcription is uncoupled from its A.RP2/3-dependent cytoplasmic office in actin polymerization. J. Immunol. 193, 150–160. doi: 10.4049/jimmunol.1302923
PubMed Abstract | CrossRef Full Text | Google Scholar
Sasahara, Y., Rachid, R., Byrne, M. J., de la Fuente, M. A., Abraham, R. T., Ramesh, N., et al. (2002). Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol. Cell ten, 1269–1281. doi: ten.1016/s1097-2765(02)00728-ane
CrossRef Full Text | Google Scholar
Sato, M., Sawahata, R., Takenouchi, T., and Kitani, H. (2011). Identification of Fyn as the binding partner for the WASP N-final domain in T cells. Int. Immunol. 23, 493–502. doi: 10.1093/intimm/dxr042
PubMed Abstruse | CrossRef Full Text | Google Scholar
Schaeffer, H. J., and Weber, Grand. J. (1999). Mitogen-activated poly peptide kinases: specific letters from ubiquitous messengers. Mol. Jail cell. Biol. 19, 2435–2444. doi: x.1128/mcb.xix.4.2435
PubMed Abstruse | CrossRef Full Text | Google Scholar
Schamel, Due west. W., Alarcon, B., and Minguet, South. (2019). The TCR is an allosterically regulated macromolecular machinery changing its conformation while working. Immunol. Rev. 291, 8–25. doi: 10.1111/imr.12788
PubMed Abstract | CrossRef Full Text | Google Scholar
Silvin, C., Belisle, B., and Abo, A. (2001). A part for Wiskott-Aldrich syndrome poly peptide in T-prison cell receptor-mediated transcriptional activation independent of actin polymerization. J. Biol. Chem. 276, 21450–21457. doi: 10.1074/jbc.M010729200
PubMed Abstract | CrossRef Full Text | Google Scholar
Lord's day, Z., Arendt, C. West., Ellmeier, Westward., Schaeffer, E. G., Sunshine, M. J., Gandhi, L., et al. (2000). PKC-theta is required for TCR-induced NF-kappa B activation in mature but not young T lymphocytes. Nature 404, 402–407. doi: 10.1038/35006090
PubMed Abstract | CrossRef Full Text | Google Scholar
Swamy, M., Beck-Garcia, One thousand., Beck-Garcia, E., Hartl, F. A., Morath, A., Yousefi, O. S., et al. (2016). A cholesterol-based allostery model of T cell receptor phosphorylation. Immunity 44, 1091–1101. doi: 10.1016/j.immuni.2016.04.011
PubMed Abstract | CrossRef Total Text | Google Scholar
Symons, M., Derry, J. M., Karlak, B., Jiang, Southward., Lemahieu, V., Mccormick, F., et al. (1996). Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell 84, 723–734. doi: ten.1016/s0092-8674(00)81050-8
CrossRef Full Text | Google Scholar
Thumkeo, D., Katsura, Y., Nishimura, Y., Kanchanawong, P., Tohyama, K., Ishizaki, T., et al. (2020). mDia1/3-dependent actin polymerization spatiotemporally controls LAT phosphorylation by Zap70 at the immune synapse. Sci. Adv. six:eaay2432. doi: 10.1126/sciadv.aay2432
PubMed Abstract | CrossRef Full Text | Google Scholar
Torres, E., and Rosen, Thou. M. (2006). Protein-tyrosine kinase and GTPase signals cooperate to phosphorylate and activate Wiskott-Aldrich syndrome protein (WASP)/neuronal WASP. J. Biol. Chem. 281, 3513–3520. doi: 10.1074/jbc.M509416200
PubMed Abstract | CrossRef Full Text | Google Scholar
Trifari, S., Sitia, G., Aiuti, A., Scaramuzza, S., Marangoni, F., Guidotti, L. G., et al. (2006). Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott-Aldrich syndrome patients. J. Immunol. 177, 7451–7461. doi: 10.4049/jimmunol.177.ten.7451
PubMed Abstract | CrossRef Full Text | Google Scholar
Volkman, B. F., Prehoda, Thousand. Due east., Scott, J. A., Peterson, F. C., and Lim, W. A. (2002). Construction of the Due north-WASP EVH1 domain-WIP complex: insight into the molecular basis of Wiskott-Aldrich Syndrome. Cell 111, 565–576. doi: 10.1016/s0092-8674(02)01076-0
CrossRef Total Text | Google Scholar
Watanabe, Y., Sasahara, Y., Ramesh, Due north., Massaad, M. J., Yeng Looi, C., Kumaki, South., et al. (2013). T-prison cell receptor ligation causes Wiskott-Aldrich syndrome protein degradation and F-actin assembly downregulation. J. Allergy Clin. Immunol. 132, 648–655.e1. doi: ten.1016/j.jaci.2013.03.046
PubMed Abstract | CrossRef Full Text | Google Scholar
Wipa, P., Paensuwan, P., Ngoenkam, J., Woessner, N. Yard., Minguet, S., Schamel, Due west. W., et al. (2020). Actin polymerization regulates recruitment of Nck to CD3ε upon T-prison cell receptor triggering. Immunology 159, 298–308. doi: 10.1111/imm.13146
PubMed Abstract | CrossRef Full Text | Google Scholar
Wunderlich, L., Faragó, A., Downward, J., and Buday, Fifty. (1999). Clan of Nck with tyrosine-phosphorylated SLP-76 in activated T lymphocytes. Eur. J. Immunol. 29, 1068–1075. doi: x.1002/(SICI)1521-4141(199904)29:04<1068::Aid-IMMU1068<3.0.CO;2-P
CrossRef Full Text | Google Scholar
Yablonski, D., Kane, L. P., Qian, D., and Weiss, A. (1998). A Nck-Pak1 signalling module is required for T-prison cell receptor-mediated activation of NFAT, but not of JNK. EMBO J. 17, 5647–5657. doi: x.1093/emboj/17.nineteen.5647
PubMed Abstract | CrossRef Total Text | Google Scholar
Yokosuka, T., and Saito, T. (2010). "The immunological synapse, TCR microclusters, and T cell activation," in Immunological Synapse. Current Topics in Microbiology and Immunology, vol 340, eds T. Saito and F. Batista (Berlin: Spinger), doi: 10.1007/978-3-642-03858-7_5
PubMed Abstract | CrossRef Total Text | Google Scholar
Zeng, R., Cannon, J. L., Abraham, R. T., Way, M., Billadeau, D. D., Bubeck-Wardenberg, J., et al. (2003). SLP-76 coordinates Nck-dependent Wiskott-Aldrich syndrome poly peptide recruitment with Vav-1/Cdc42-dependent Wiskott-Aldrich syndrome protein activation at the T cell-APC contact site. J. Immunol. 171, 1360–1368. doi: ten.4049/jimmunol.171.three.1360
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, J., Dong, B., and Siminovitch, Yard. A. (2009). Contributions of Wiskott-Aldrich syndrome family cytoskeletal regulatory adapters to immune regulation. Immunol. Rev. 232, 175–194. doi: 10.1111/j.1600-065X.2009.00846.ten
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zhang, J., Shehabeldin, A., da Cruz, L. A., Butler, J., Somani, A. K., McGavin, M., et al. (1999). Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J. Exp. Med. 190, 1329–1342. doi: 10.1084/jem.190.nine.1329
PubMed Abstract | CrossRef Total Text | Google Scholar
Zhang, W., Sloan-Lancaster, J., Kitchen, J., Trible, R. P., and Samelson, 50. E. (1998). LAT: the ZAP-seventy tyrosine kinase substrate that links T prison cell receptor to cellular activation. Cell 92, 83–92. doi: x.1016/s0092-8674(00)80901-0
CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fcell.2021.674572/full
Post a Comment for "What Family of Proteins Do Tcr Belong to?"